Explain Why All Three Net-ionic Equations Are Different
Explain why it is valid to remove this species from the equation. 4H aq.
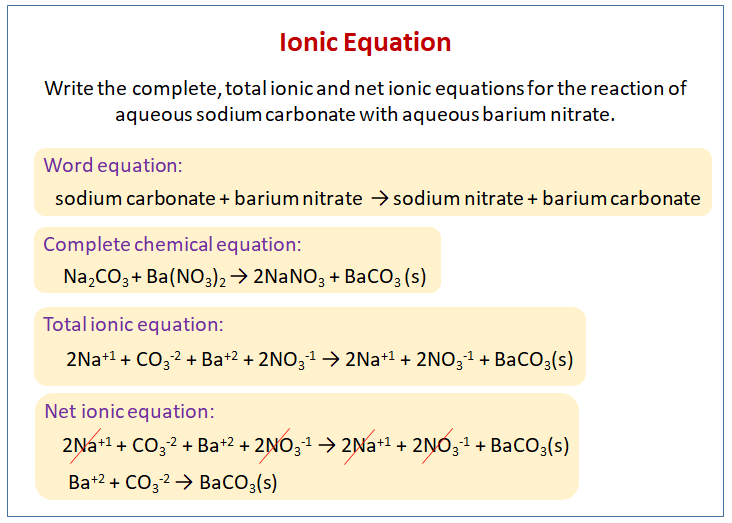
Writing Ionic Equation Video Lessons Examples And Solutions
The net ionic equation is commonly used in acid-base neutralization reactions double displacement reactions and redox reactions.
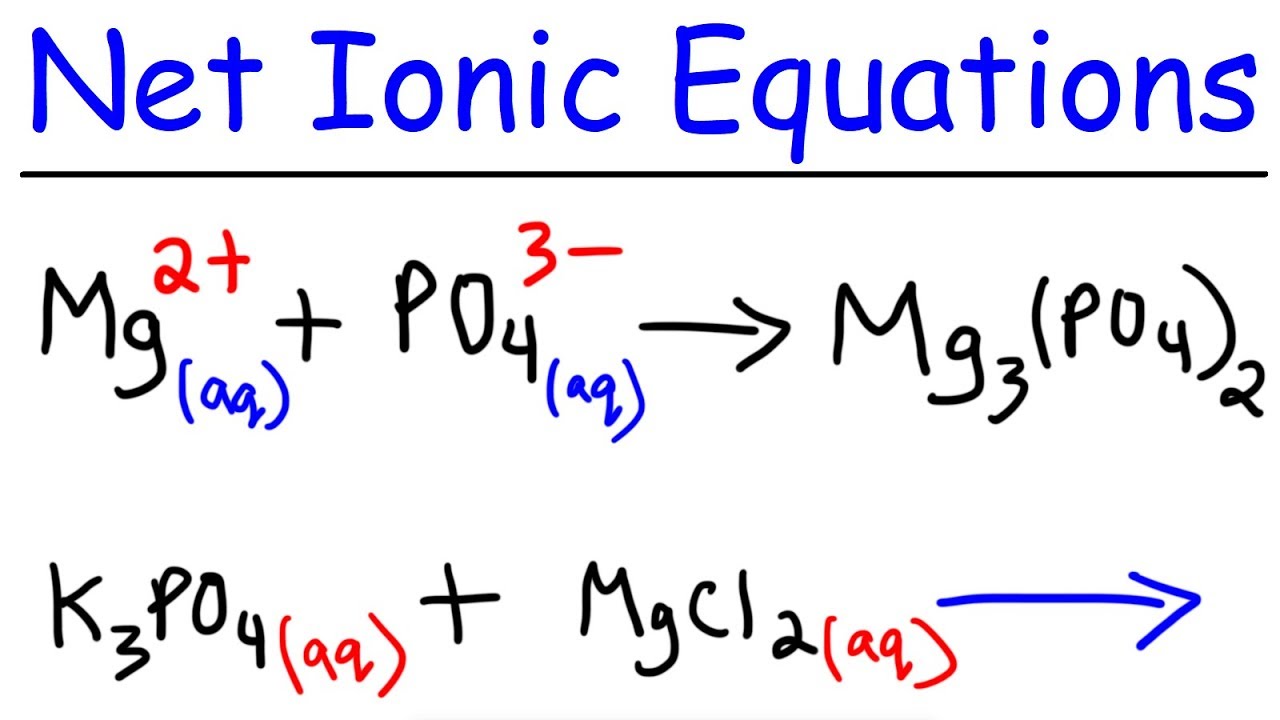
. In the complete ionic equation soluble ionic compounds and strong acids are rewritten as dissociated ions. Sid S - did no chanqt 8. So in this case H 2 SO 4 aq and Ba OH 2 aq must be.
It is the process by which one or more substances change into one or more new substances that have new chemical and physical properties. Then net ionic equation is formed. The net ionic equation is a chemical equation for a reaction that lists only those species participating in the reaction.
I leave out sodium and chloride ions because they are irrelevant. Your net ionic equation leaves out spectator ions and focuses on what changes in the reaction. One of the equations represents an acid-base reaction.
October 13 2019 Posted by Madhu. Review net-ionic equations 282 284 287289 and 2811. Work as a group to write a definition for a net ionic equation.
Be familiar with the experimental steps and what was observed in the experiment in which crystals of silver nitrate and potassium chloride were pushed into a puddle Section D. Copper forms many different compounds. The complete ionic equation indicates all of the dissociated ions in a chemical reaction.
Experiments 5 9 and 11 are reactions of an acid with a carbonate salt to yield CO2g and H2Ol yet all have different net ionic equations. The two most common forms of ionic equations are complete ionic equations and net ionic equations. Balance the oxygen atoms.
Re-count the number of atoms on each side of the equation to make sure they are equal. In the third line the full ionic equation is repeated but cancellation of species appearing the same on both sides is indicated which leads to the final equation the net ionic equation. SPCCCS Q CL poviå- JrÁL YCQCfiovx STOP.
Explain why all three net-ionic equations in Steps 1 2 and 3 are different. Identify the equation and indicate the base. In the net ionic equation any ions that do not participate in the reaction called spectator ions are.
Then write the ionic equation showing all aqueous substances as ions. Identify the three equations and indicate the oxidiz- ing agent in each. The key difference between molecular equation and ionic equation is that the molecular equation shows the reactants and products in molecular form while the ionic equation shows the ionic species involved in the reaction.
A net ionic equation is a reaction equation in which all spectator ions are removed leaving only the ions and molecules directly involved in the reaction. What is the net ionic equation between HNO 3 aq and TiOH 4 s. Add a coefficient in front of elements that are not oxygen and hydrogen to balance each side.
The result of making all these replacements is the complete ionic equation. Calculate the molar solubility of CdCO3 in a buffer solution containing 0115 M Na2CO3 and 0120 M. When spectator ions are cancel from the both side of the ionic equation.
In the molecular equation for a reaction all of the reactants and products are represented as neutral molecules even soluble ionic compounds and strong acids. Your complete ionic equation includes all ions in solution including spectator ions. Different mole ratios occur for other polyprotic acids or bases with multiple hydroxides such as CaOH 2.
The net ionic equation cancels out ions that appear on both sides of the reaction arrow because they essentially dont participate in the reaction of interest. For example Cr NiCl 2 -- CrCl 3 Ni becomes 2Cr 3NiCl 2 -- 2CrCl 3 3Ni. The sodium sulfate salt is soluble and so the net ionic reaction is again the same.
In order to write the net ionic equation the weak acid must be written as a molecule since it does not ionize to a great extent in water. Finally eliminate spectator ions. Net ionic equation is Ba2.
Why are the net ionic equations different for the acid base reactions numbers 1 and 12. APPLICATION OF PRINCIPLES Classify each of the following reactions as 1 combination 2 decomposition 3 displacement or 4 metathesis. Up to 24 cash back 7.
Compare the net ionic equation in Model 2 to the other two equations. Chemical reactions are interactions between chemical compounds to form new compounds or. In other words the net ionic equation applies to reactions that are strong electrolytes in.
What chemical species is missing in the net ionic equation. Place the appropriate number in the blank at the left of the equation. Net ionic equation by removing the spectator ions because spectator ions are present in both side of the ionic equation.
What is a reactant. Balance the hydrogen atoms. H 2 SO 4 aq Ba OH 2 aq --- BaSO 4 s 2H 2 O l So the molecular form of the equation is shown above.
Complete ionic equation and net ionic equation are two ways of representing a chemical reaction. To write the ionic equation we must separate all aqueous species into their ions and leave any solid liquid or gaseous substance in its molecular form. Explain why the net ionic equation for the neutralization reaction between HClaq and KOHaq is the same as the net ionic equation for the neutralization reaction between HNO3aq and RbOHaq.
We can find the net ionic equation for a given reaction using the following steps. In a chemical reaction the reactants are the original substances these can be elements or compounds. A net ionic equation shows only the chemical species that are involved in a reaction while a complete ionic equation also includes the spectator ions.
Plan the problem. Carry through any coefficients. Because nothing is dissolved there are no substances to separate into ions so the net ionic equation is the equation of the three solids and one liquid.
The key difference between Complete ionic and net ionic equation is that complete ionic equation gives all the ionic species participated in the chemical reaction whereas net ionic reaction. Students also viewed these Organic Chemistry questions. The chemical equation shows what the reactants are and what are the products.
In any chemical reaction there are reactants and products. Write and balance the molecular equation first making sure that all formulas are correct. To a 010-M solution of Pb NO32 is added enough HF g to make HF010M.
Three of the equations represent oxidation-reduction reactions. 1 N yer 3 yey Y 4 5 6 6M HC2H2O2 6M NaOH Exothermic No no 05 M Na2CO3 01M CaCl2 Excothermic. A net ionic equation is one in which spectator ions are removedMolecular equations show species reacting as their molecular formula with subscripts added to indicate their solid liquid gaseous or aqueous nature.
Write the balanced molecular equation for the reaction including the state of each substance. For the neutralization reaction between any monoprotic strong acid and strong base the resulting net. Calculate the molar solubility of BaF2 in a buffer solution containing 020 M HF and 020 M NaF.

How To Write Net Ionic Equations In Chemistry A Simple Method Youtube
Precipitation Reactions Net Ionic Equations Chemistry Youtube

Difference Between Molecular Equation And Ionic Equation Compare The Difference Between Similar Terms

Comments
Post a Comment